ABC Heart Fail Cardiomyop 2025; 5(2): e20240052
Genetic Cardiomyopathies: An Overview of the Main Associated Pathogenic Mutations
Abstract
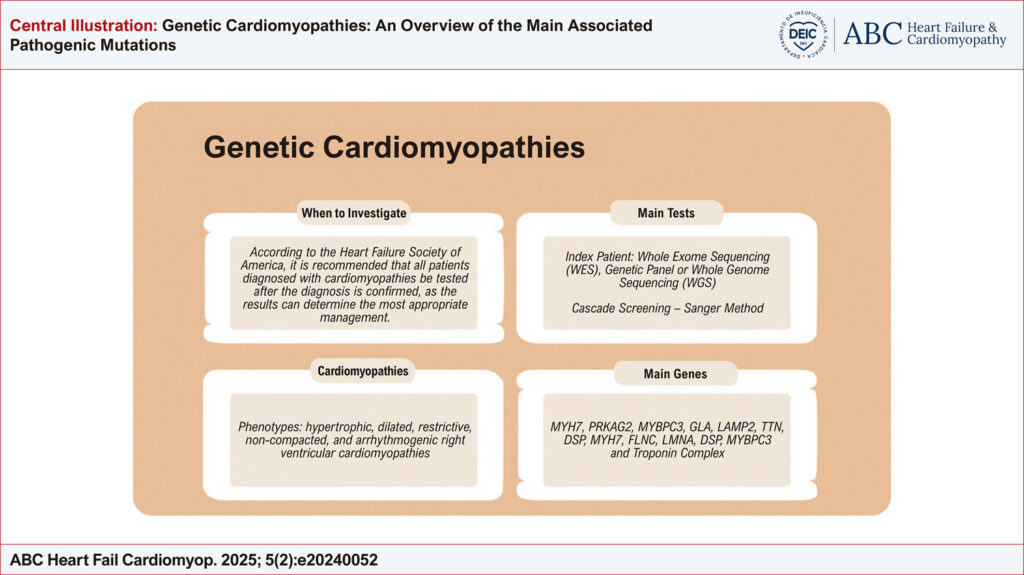
Cardiomyopathies are pathological conditions associated with progressive dysfunction of cardiomyocytes, excluding situations in which other concomitant diseases may justify such progression. Their presentations can evolve with several phenotypes, the main ones being hypertrophic, dilated, restrictive, non-compacted, and arrhythmogenic phenotypes of the right ventricle (). Regarding this topic’s relevance, dilated and hypertrophic cardiomyopathies have a genetic origin in 30 to 50% of cases, evaluating these etiologies is a crucial tool in patient management. Assessing the risk of genetic transmission and the likelihood of phenotypic manifestation across generations requires identifying and classifying each gene’s inheritance pattern and mutation type associated with structural cardiomyopathy. Additionally, determining the structural protein affected facilitates understanding the pathophysiological phenomenon and the phenotype manifested by the variant carrier. Considering the growing advent of the genetic approach to cardiomyopathies, the Heart Failure Society of America, in collaboration with the American College of Medical Genetics and Genomics, developed a guide for the investigation and follow-up of these cases. However, in this study, the time to request the genetic tests was not evaluated. It is recommended that the tests be requested at any time after diagnosis confirmation since their results can contribute to guiding the most appropriate follow-up approach. Despite the multitude of existing variants related to genetic cardiomyopathies, some are of greater clinical interest due to their impact on risk stratification and interest in family screening, namely: MYH7, PRKAG2, MYBPC3, GLA, LAMP2, TTN, DSP, MYH7, FLNC, LMNA, DSP, MYBPC3 and Troponin Complex (). Recognizing the main mutations associated with cardiomyopathies and the phenotypes related to them enables a more individualized medicine approach with early interventions and risk stratification. Genetic evaluation is essential for screening family members and optimizing patient management, which may vary depending on the identified mutation.
370