ABC Heart Fail Cardiomyop 2022; 2(4): 415-416
Ventricular Dysfunction and Heart Failure in Patients Undergoing Hematopoietic Stem Cell Transplant
It has been known for a long time that Cyclophosphamide (Cy) for conditioning regimens in hematopoietic stem cell transplant (HSCT) can induce cardiotoxicity. We have been learning in cardio-oncology that the best practice is to identify the risks of cardiotoxicity and, when possible, minimize them before the exposure. Several factors contribute to define cardiovascular toxicity risks including the type of transplant, age at transplant, hypertension, diabetes mellitus, dyslipidemia, and current smoking. Multiple uncontrolled preexisting cardiovascular conditions such as atrial fibrillation or atrial flutter, node sinus disease, ventricular arrhythmias, coronary artery disease, valve diseases and heart failure with reduced ejection fraction may also contribute to cardiotoxicity. So, it is important to know about the disease severity at the time of transplantation and probability of relapse, which may be correlated with increased exposure to other cardiotoxic chemotherapy drugs and radiotherapy before HSCT.
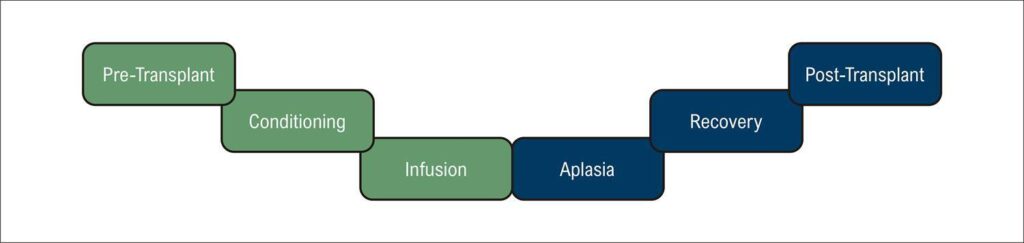
There are mainly two types of HSCT: (1) autologous transplantation, in which the patient receives his/her own progenitor cells, and the cardiotoxicity is mainly attributed to the direct toxic effects of the conditioning regimen; and (2) allogeneic transplantation in which the patient receives cells from another individual who may be fully or partially compatible. The allogeneic transplantation is known to have a higher risk of cardiotoxicity because in addition to the conditioning toxic effects, there are indirect effects of cytokines released in graft-versus-host disease and a possible direct cardiotoxicity through donor T-cell infiltration into the myocardium with subsequent inflammation leading to cardiac dysfunction. The HSCT has six stages as presented in : pre-transplant, conditioning phase (when the patient receives chemotherapy and often radiotherapy to eliminate the recipient’s bone marrow, infusion, aplasia, recovery and post-transplant stage. In pre-transplant, the cardio-oncologist must perform a comprehensive assessment of the risk factors for cardiotoxicity. Clinical history and physical examination, electrocardiogram, laboratory tests including T troponin and brain natriuretic peptide (BNP) (or N-terminal-proBNP NTproBNP), X-ray or, preferably, chest computed tomography, strain echocardiogram and spirometry test are essentials for a good evaluation. Rhythm and hemodynamic changes are described in the infusion stage, usually quick and benign as sinus tachycardia or bradycardia, hypotension or hypertension, but also serious ones as a total AV block. These changes seem to be caused by dimethyl sulfoxide, a cryoprotectant, but there is still not enough data to support this hypothesis. Many infectious complications may occur in the aplasia phase, such as myocarditis due to viral reactivation and sepsis-induced cardiomyopathy. The differential diagnosis between these conditions and cardiotoxicity can be challenging.
[…]
Keywords: Cardiotoxicity; Heart Failure; Medula Óssea; Transplant
261