ABC Heart Fail Cardiomyop 2022; 2(4): 404-409
CAR-T Cells Therapy: What Cardiovascular Adverse Effects Should We Expect?
Abstract
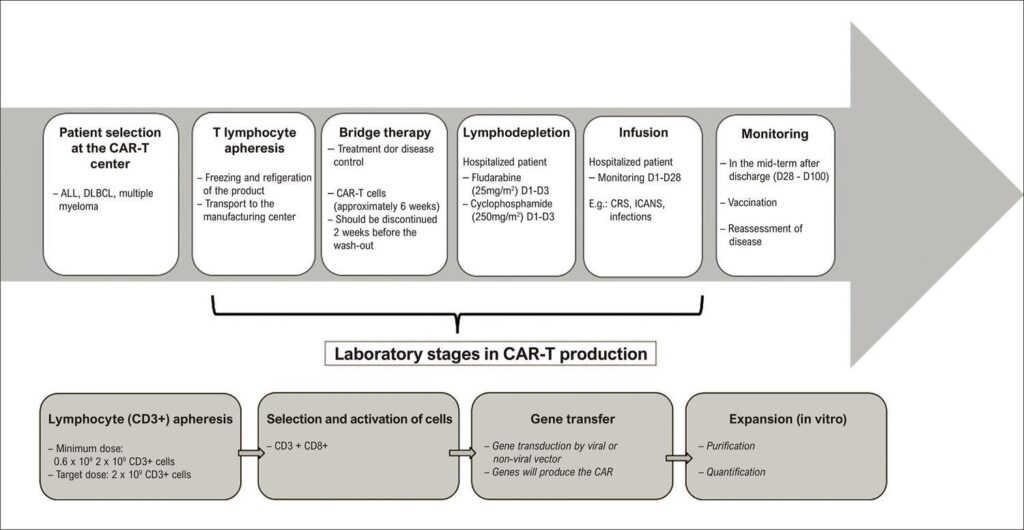
Immunotherapy has emerged as a specific treatment, guided by oncologic targets, that has given the false impression (to oncologists and hematologists) that it had no adverse effects, including cardiovascular complications. Chimeric antigen receptor (CAR) T-cell therapy has certainly benefited specific patients; in contrast, reports of adverse cardiovascular effects have emerged. In this paper, we conducted a narrative review of conceptual aspects, clinical applicability, the cytokine release syndrome, extracardiac AE and, above all, the cardiovascular AE of CAR-T cell therapy. CAR-T cell therapy has been approved by regulatory agencies in the United States, Europe and Brazil, initially for the treatment of hematological malignancies at advanced stages, after failure or refractoriness. Among extracardiac AE, cytokine release syndrome and, consequently, encephalopathy, macrophage activation syndrome or hemophagocytic lymphohistiocytosis stand out. The most frequently described cardiovascular AE are cardiomyopathies, myocarditis and ventricular dysfunction; tachyarrhythmias, changes in QT and other electrocardiographic changes; pleural and pericardial disease, and changes in biomarkers. Thus, prior to the therapy, it is recommended to perform clinical evaluation, electrocardiogram, echocardiogram, and troponin and B-type natriuretic peptide measurements. The patient should be monitored during treatment with continuous electrocardiogram, serial echocardiogram, and biomarkers. Patients with severe cardiovascular AE should be admitted to a cardiac intensive care unit.
323